
AET – What is it?
Biocompatibility is currently one of the hottest topics in medical device technology. An essential component for biological safety assessment is the chemical characterization of detachable substances. The approach is described in ISO 10993-18, which was last updated in 2020.
After adaptation of ISO 10993-18 in 2020 on the chemical characterization of materials used in medical devices, the term AET was introduced. We want to explain what AET means and how it impacts the biocompatibility review.
What does AET stand for?
The AET is a term used in analytics and defines the concentration above extractable substances that needs to be identified and toxicologically evaluated. In other words, below this value, the risk of systemic toxic effects is judged negligible.
Watch the video on the blogpost
[borlabs-cookie id="youtube" type="content-blocker"][/borlabs-cookie]
The calculation of the AET
The basis for the derivation of the AET is the so-called TTC concept or "Threshold of Toxicological Concern" This concept originates from the food sector. Here, a large number of organic substances with different chemical structures and toxicological potentials were examined in order to derive default threshold values below which the risk of systemic effects or genotoxic effects is negligible. In this context, genotoxicity (mutagenic substances) is considered the most sensitive endpoint.
In addition to the TTC limit, data on clinical use (how many products at a time, duration of use) and analytical methodology (product quantities, extraction volume, uncertainty factors of the analytical method) are also required. The calculation formula then looks as follows:
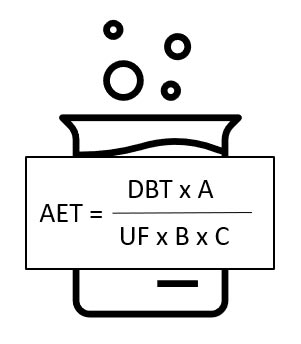
Where DBT is the dose-based threshold, A is the number of medical devices extracted, UF is the uncertainty factor of the analytical method, B is the volume of extract, and C is the clinical exposure.
Facts around the AET
What data do I need to provide to the lab or service provider to estimate an AET?
- Product sizes that are used in the clinic practice
- Maximum number of products that will be used simultaneously
- Duration of use and, if applicable, frequency of changing
What data are needed from the laboratory?
- The volume of extraction
- The amount of devices extracted
- The uncertainty factor for the analytical method
For different device types, it may also occur that the AET needs to be calculated based on the contact area or mass rather than the number of products.
Why should I hand over the AET calculation to experienced persons?
- The selection of the correct TTC value is subject to certain conditions (not permitted for all organic substances)
- The decision has to be made whether the calculation is based on device number, surface area or mass of the product
- If necessary, the DBT needs to be adapted to the relevant patient collective
The AET in biocompatibility assessment
Finally, there is the question of the impact of the AET value on the overall biological evaluation. With the introduction of the AET value, many laboratories found out that their detection limits for analytical methods for screening of soluble substances were too high, especially for products with a high contact surface. Therefore, the test methods were adapted accordingly, for example by concentrating the extracts.
What are the consequences of adapting the experimental methodology?
- Concentration of the extracts can lead to precipitation of substances.
- Concentration may result in the formation of degradation products which are not formed under physiological conditions.
- Volatile substances may be lost due to the concentration.
- The higher sensitivity of the measurement method leads to the increased number of detected substances that needs to be evaluated toxicologically.
In summary, the AET value has become essential in chemical characterization of organic substances. The introduction of the analytical threshold value has led to a higher sensitivity of analytical screening methods, which ultimately culminates in an increased effort in the toxicological evaluation of extractable substances.






